Human evolution, the process by which human beings developed on Earth from now-extinct primates. Viewed zoologically, we humans are Homo sapiens, a culture-bearing upright-walking species that lives on the ground and very likely first evolved in Africa about 315,000 years ago. We are now the only living members of what many zoologists refer to as the human tribe, Hominini, but there is abundant fossil evidence to indicate that we were preceded for millions of years by other hominins, such as Ardipithecus, Australopithecus, and other species of Homo, and that our species also lived for a time contemporaneously with at least one other member of our genus, H. neanderthalensis (the Neanderthals). In addition, we and our predecessors have always shared Earth with other apelike primates, from the modern-day gorilla to the long-extinct Dryopithecus. That we and the extinct hominins are somehow related and that we and the apes, both living and extinct, are also somehow related is accepted by anthropologists and biologists everywhere. Yet the exact nature of our evolutionary relationships has been the subject of debate and investigation since the great British naturalist Charles Darwin published his monumental books On the Origin of Species (1859) and The Descent of Man (1871). Darwin never claimed, as some of his Victorian contemporaries insisted he had, that “man was descended from the apes,” and modern scientists would view such a statement as a useless simplification—just as they would dismiss any popular notions that a certain extinct species is the “missing link” between humans and the apes. There is theoretically, however, a common ancestor that existed millions of years ago. This ancestral species does not constitute a “missing link” along a lineage but rather a node for divergence into separate lineages. This ancient primate has not been identified and may never be known with certainty, because fossil relationships are unclear even within the human lineage, which is more recent. In fact, the human “family tree” may be better described as a “family bush,” within which it is impossible to connect a full chronological series of species, leading to Homo sapiens, that experts can agree upon.
The primary resource for detailing the path of human evolution will always be fossil specimens. Certainly, the trove of fossils from Africa and Eurasia indicates that, unlike today, more than one species of our family has lived at the same time for most of human history. The nature of specific fossil specimens and species can be accurately described, as can the location where they were found and the period of time when they lived; but questions of how species lived and why they might have either died out or evolved into other species can only be addressed by formulating scenarios, albeit scientifically informed ones. These scenarios are based on contextual information gleaned from localities where the fossils were collected. In devising such scenarios and filling in the human family bush, researchers must consult a large and diverse array of fossils, and they must also employ refined excavation methods and records, geochemical dating techniques, and data from other specialized fields such as genetics, ecology and paleoecology, and ethology (animal behaviour)—in short, all the tools of the multidisciplinary science of paleoanthropology.
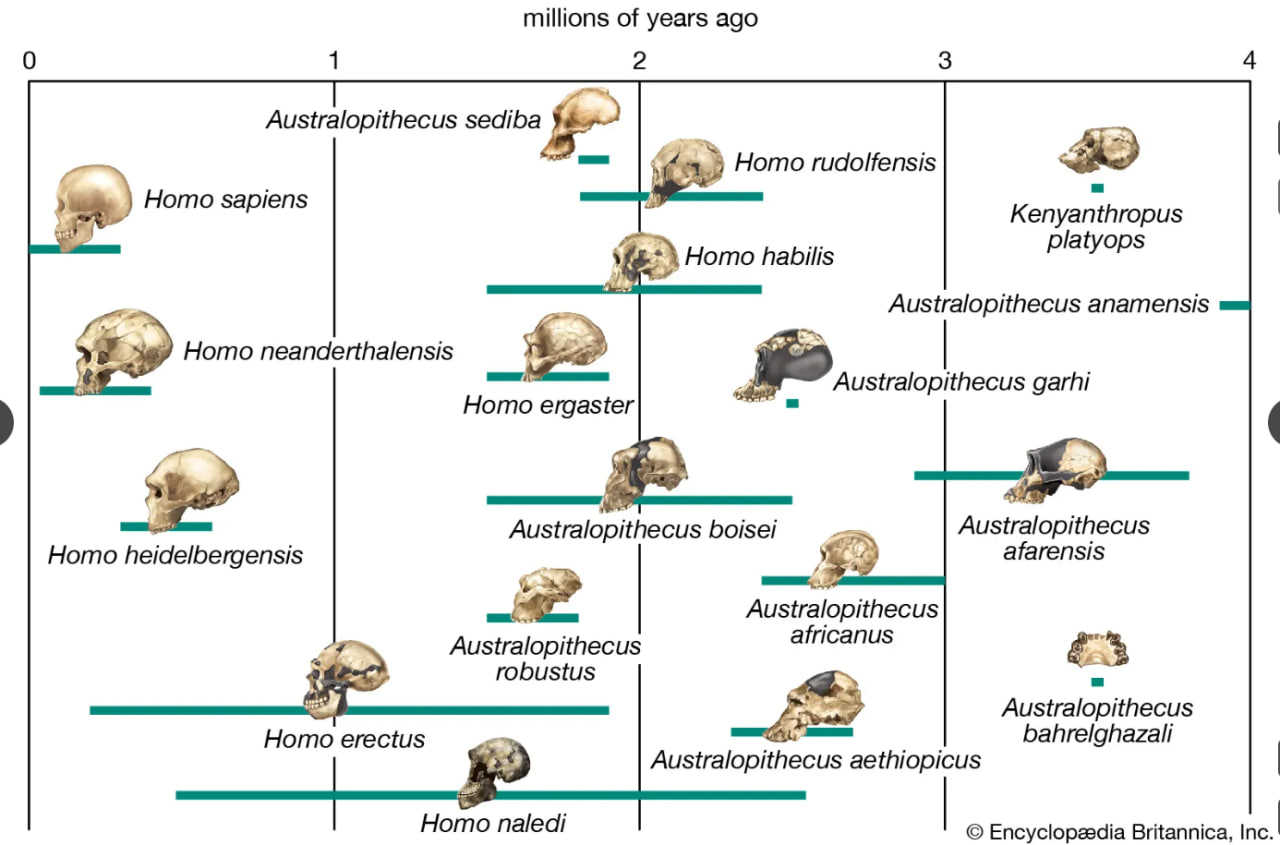
human evolutionary pathways
Possible pathways in the evolution of the human lineage.
This article is a discussion of the broad career of the human tribe from its probable beginnings millions of years ago in the Miocene Epoch (23 million to 5.3 million years ago [mya]) to the development of tool-based and symbolically structured modern human culture only tens of thousands of years ago, during the geologically recent Pleistocene Epoch (about 2.6 million to 11,700 years ago). Particular attention is paid to the fossil evidence for this history and to the principal models of evolution that have gained the most credence in the scientific community.See the article evolution for a full explanation of evolutionary theory, including its main proponents both before and after Darwin, its arousal of both resistance and acceptance in society, and the scientific tools used to investigate the theory and prove its validity.
Background and beginnings in the Miocene
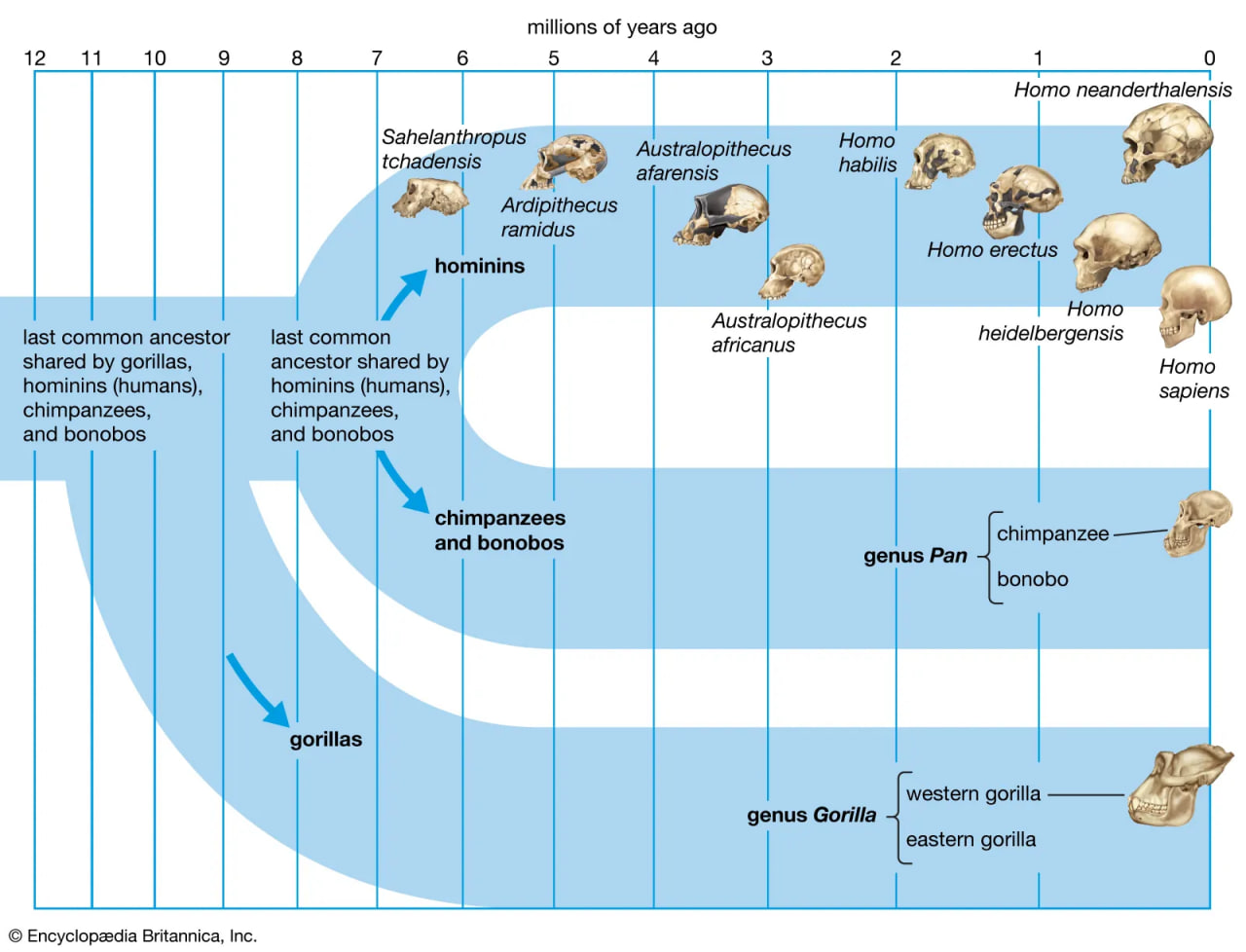
human evolution
The divergence of humans and great apes from a common ancestor.
It is generally agreed that the taproot of the human family shrub is to be found among apelike species of the Middle Miocene Epoch (roughly 16–11.6 mya) or Late Miocene Epoch (11.6–5.3 mya). Genetic data based on molecular clock estimates support a Late Miocene ancestry. Various Eurasian and African Miocene primates have been advocated as possible ancestors to the early hominins, which came on the scene during the Pliocene Epoch (5.3–2.6 mya). Though there is no consensus among experts, the primates suggested include Kenyapithecus, Griphopithecus, Dryopithecus, Graecopithecus (Ouranopithecus), Samburupithecus, Sahelanthropus, and Orrorin. Kenyapithecus inhabited Kenya and Griphopithecus lived in central Europe and Turkey from about 16–14 mya. Dryopithecus is best known from western and central Europe, where it lived from 13 to possibly 8 mya. Graecopithecus lived in northern and southern Greece about 9 mya, at roughly the same time as Samburupithecus in northern Kenya. Sahelanthropus inhabited Chad between 7 and 6 million years ago. Orrorin was from central Kenya 6 mya. Among these, the most likely ancestor of great apes and humans may be either Kenyapithecus or Griphopithecus.
Among evolutionary models that stress the Eurasian species, some consider Graecopithecus to be ancestral only to the human lineage, containing Australopithecus, Paranthropus, and Homo, whereas others entertain the possibility that Graecopithecus is close to the great-ape ancestry of Pan (chimpanzees and bonobos) and Gorilla as well. In the former model, Dryopithecus is ancestral to Pan and Gorilla. On the other hand, others would have Dryopithecus ancestral to Pan and Australopithecus on the way to Homo, with Graecopithecus ancestral to Gorilla. This morphology-based model mirrors results of some molecular studies, which show chimpanzees, bonobos, and humans to be more closely related to one another than any of them is to gorillas; orangutans (Pongo) are more distantly related.
In a phylogenetic model that emphasizes African Miocene species, Samburupithecus is ancestral to Australopithecus, Paranthropus, and Orrorin, and Orrorin begets Au. afarensis, which is ancestral to Homo.
The Miocene Epoch was characterized by major global climatic changes that led to more seasonal conditions with increasingly colder winters north of the Equator. By the Late Miocene, in many regions inhabited by apelike primates, evergreen broad-leaved forests were replaced by open woodlands, shrublands, grasslands, and mosaic habitats, sometimes with denser-canopied forests bordering lakes, rivers, and streams. Such diverse environments stimulated novel adaptations involving locomotion in many types of animals, including primates. In addition, there were a larger variety and greater numbers of antelope, pigs, monkeys, giraffes, elephants, and other animals for adventurous hominins to scavenge and perhaps kill. But large cats, dogs, and hyenas also flourished in the new environments; they not only would provide meat for scavenging hominins but also would compete with and probably prey upon them. In any case, our ancestors were not strictly or even heavily carnivorous. Instead, a diet that relied on tough, abrasive vegetation, including seeds, stems, nuts, fruits, leaves, and tubers, is suggested by primate remains bearing large premolar and molar teeth with thick enamel.
Behaviour and morphology associated with locomotion also responded to the shift from arboreal to terrestrial life. The development of bipedalism enabled hominins to establish new niches in forests, closed woodlands, open woodlands, and even more open areas over a span of at least 4.5 million years. Indeed, obligate terrestrial bipedalism (that is, the ability and necessity of walking only on the lower limbs) is the defining trait required for classification in the human tribe, Hominini.
Striding through the Pliocene
The anatomy of bipedalism
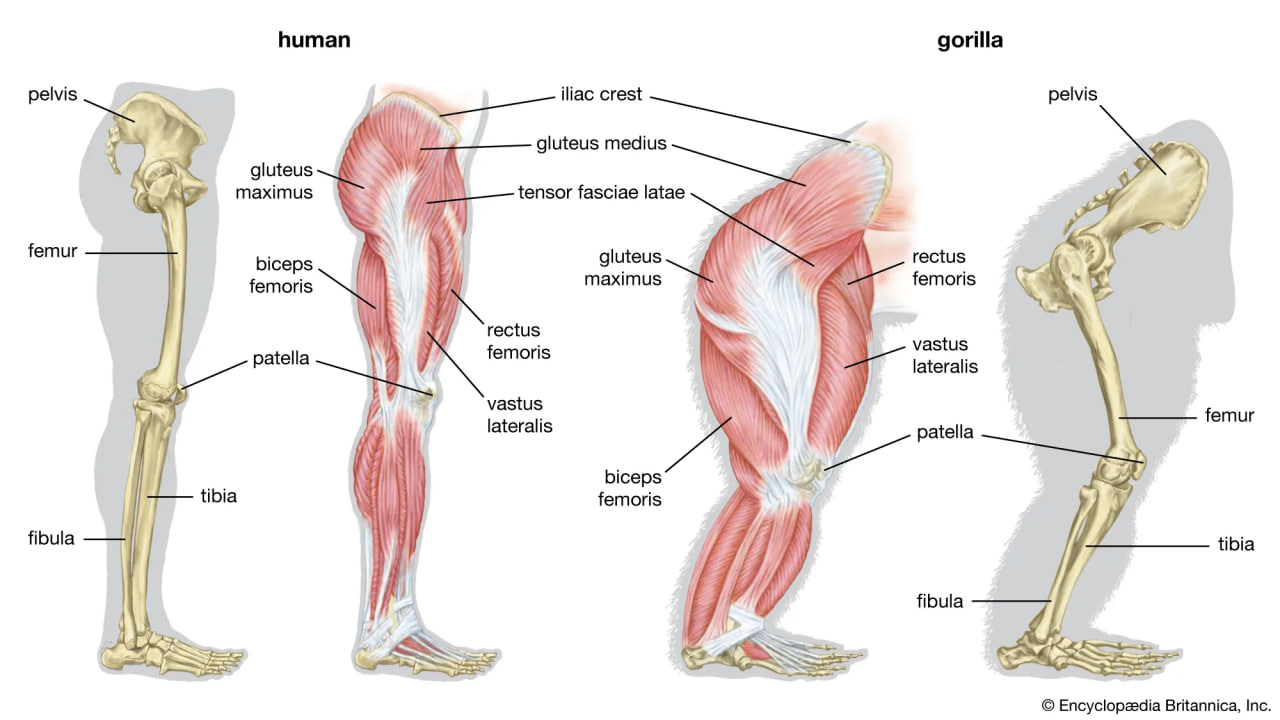
Skeletal and muscular structures of a human leg (left) and a gorilla leg (right).
Bipedalism is not unique to humans, though our particular form of it is. Whereas most other mammalian bipeds hop or waddle, we stride. H. sapiens is the only mammal that is adapted exclusively to bipedal striding. Unlike most other mammalian orders, the primates have hind-limb-dominated locomotion. Accordingly, human bipedalism is a natural development from the basic arboreal primate body plan, in which the hind limbs are used to move about and sitting upright is common during feeding and rest.
The initial changes toward an upright posture were probably related more to standing, reaching, and squatting than to extended periods of walking and running. Human beings stand with fully extended hip and knee joints, such that the thighbones are aligned with their respective leg bones to form continuous vertical columns. To walk, one simply tilts forward slightly and then keeps up with the displaced centre of mass, which is located within the pelvis. The large muscle masses of the human lower limbs power our locomotion and enable a person to rise from squatting and sitting postures. Body mass is transferred through the pelvis, thighs, and legs to the heels, balls of the feet, and toes. Remarkably little muscular effort is expended to stand in place. Indeed, our large buttock, anterior thigh, and calf muscles are virtually unused when we stand still. Instead of muscular contraction, the human bipedal stance depends more on the way in which joints are constructed and on strategically located ligaments that hold the joints in position. Fortunately for paleoanthropologists, some bones show dramatic signs of how a given hominin carried itself, and the adaptation to obligate terrestrial bipedalism led to notable anatomic differences between hominins and great apes. These differences are readily identified in fossils, particularly those of the pelvis and lower limbs.
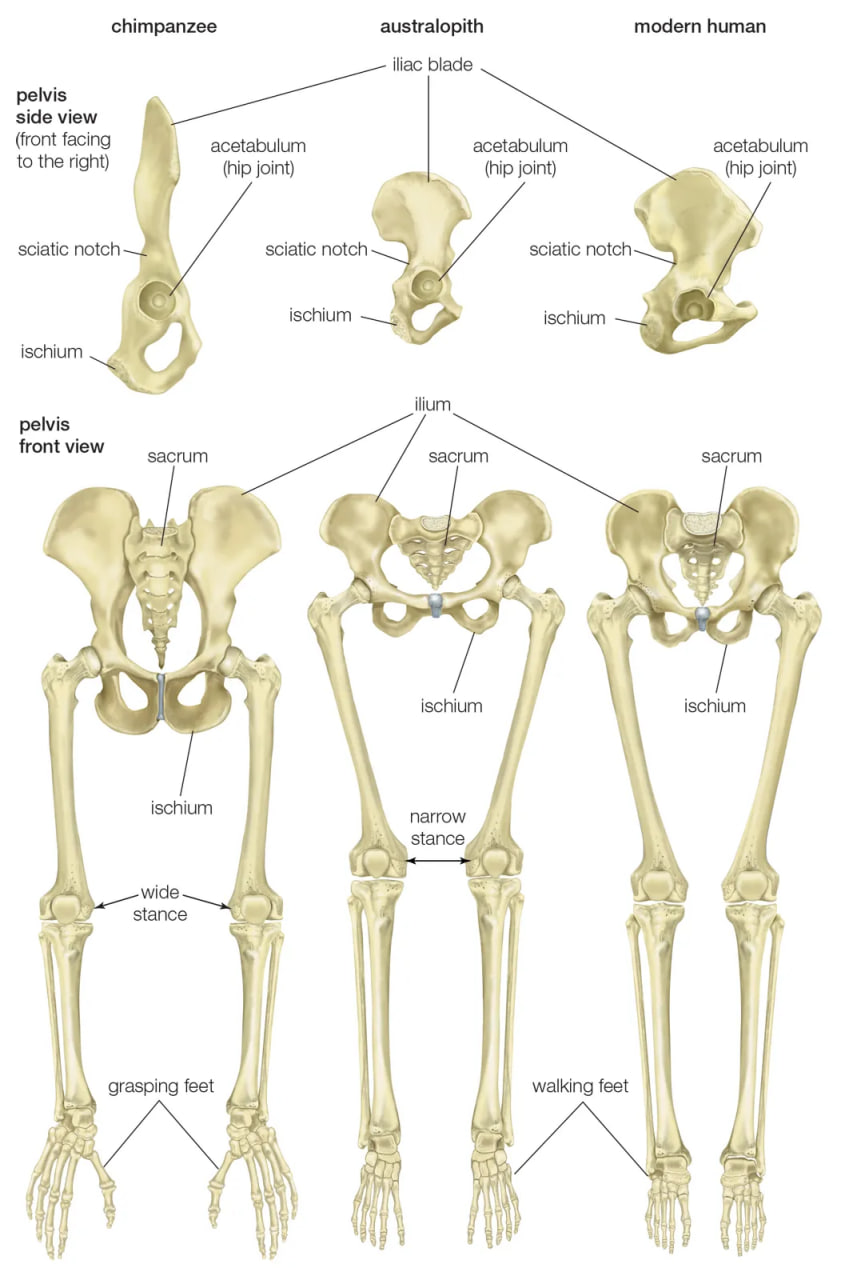
pelvis and leg bones of three great apes
Comparison of the pelvis and lower limbs of a chimpanzee, an australopith, and a modern human.
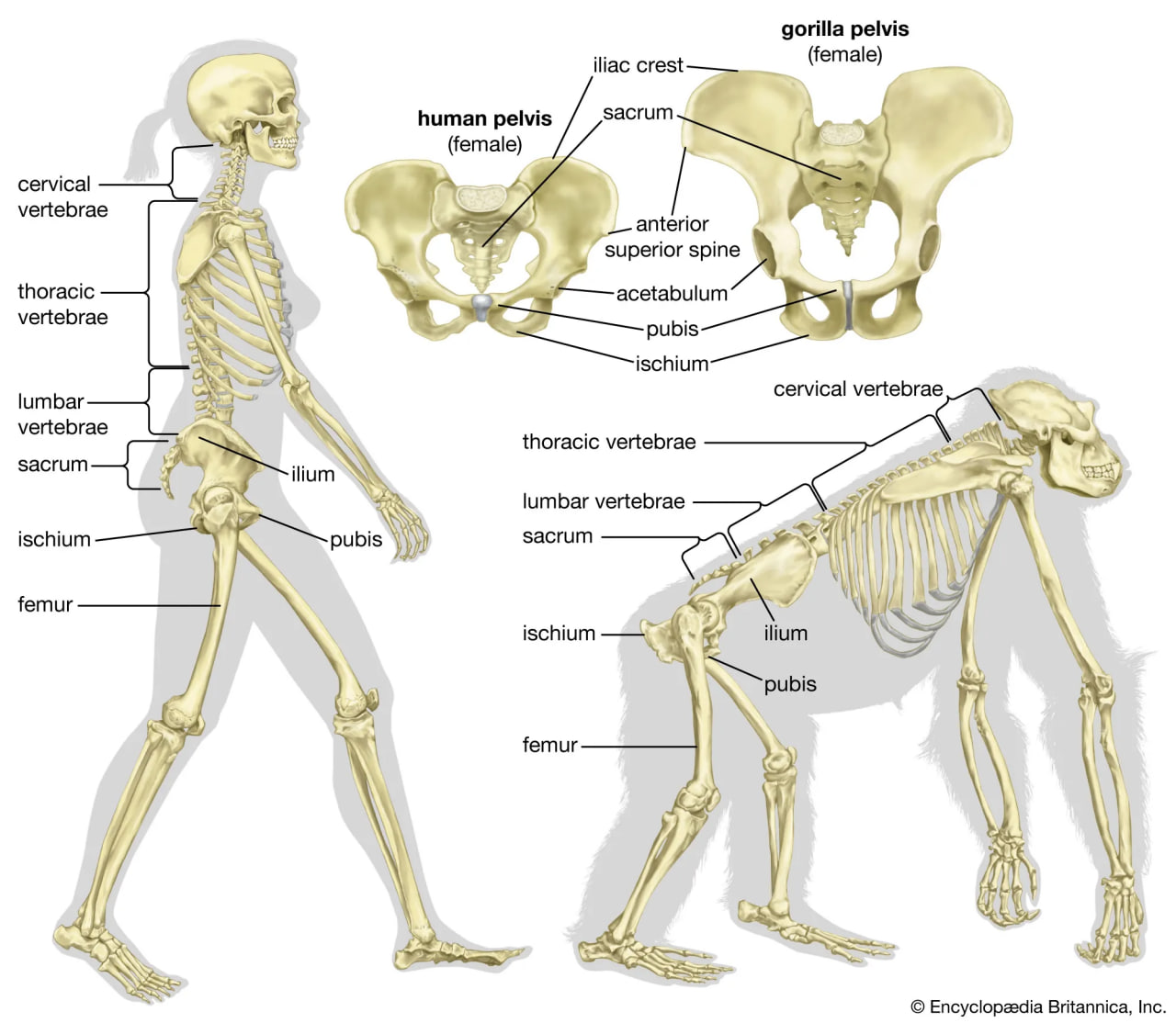
The skeletal structure of a human being (left) and of a gorilla (right).
Human feet are distinct from those of apes and monkeys. This is not surprising, since in humans the feet must support and propel the entire body on their own instead of sharing the load with the forelimbs. In humans the heel is very robust, and the great toe is permanently aligned with the four diminutive lateral toes. Unlike other primate feet, which have a mobile midfoot, the human foot possesses (if not requires) a stable arch to give it strength. Accordingly, human footprints are unique and are readily distinguished from those of other animals.
The fossil evidence

Laetoli footprints
A trail of footprints, probably left by Australopithecus afarensis individuals some 3.5 million years ago, at Laetoli, northern Tanzania
By 3.5 million years ago at least one hominin species, Au. afarensis, was an adept walker. In addition to anatomic evidence from this time, there is also a 27.5-metre (90-foot) trackway produced by three individuals who walked at a leisurely pace on moist volcanic ash at Laetoli in northern Tanzania. In all observable features of foot shape and walking pattern, they are astonishingly similar to those of habitually barefoot people who live in the tropics today. Nevertheless, although the feet of the Laetoli hominins appear to be strikingly human, one should not assume that other parts of their bodies were as similar to ours.
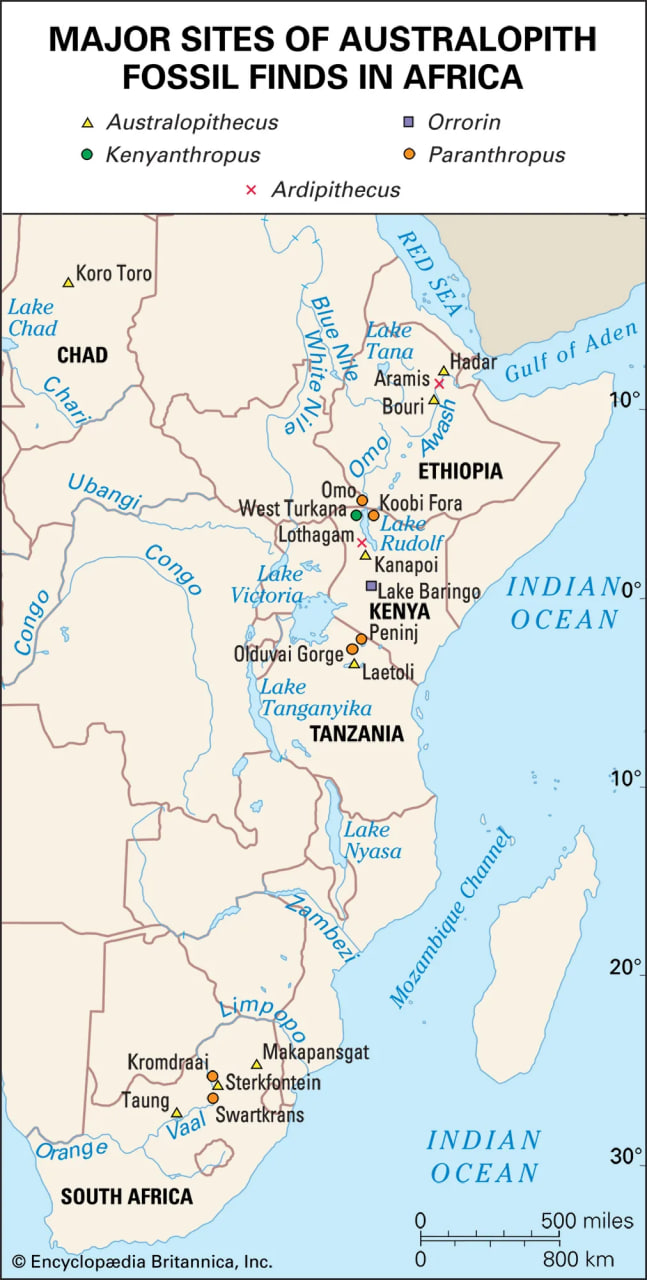
hominin fossil sites in Ethiopia
Hominin fossil sites of the Awash River basin, Ethiopia.
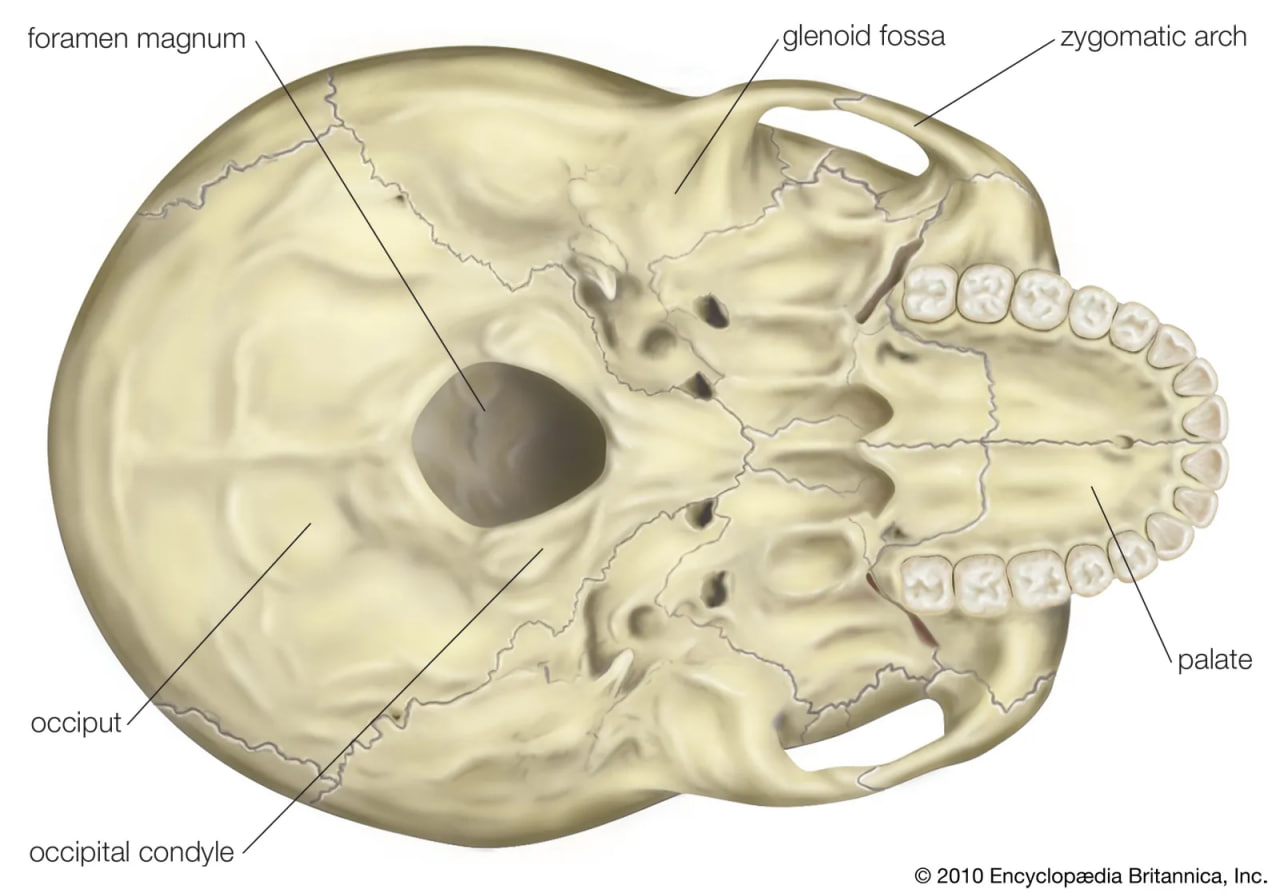
The fragmentary femoral remains found in Kenya of six-million-year-old Orrorin tugenensis indicate to some experts that they too were bipeds. Ar. ramidus (5.8–4.4 mya), a primate from Aramis, central Ethiopia, and one of the two fossil species of Ardipithecus, was also bipedal. In this case the evidence comes from the foramen magnum, the hole in the skull through which the spinal cord enters. In Ardipithecus this opening is similar to ours in being located centrally under the skull instead of at the rear of it. A rear-facing foramen magnum indicates a stooped posture, whereas a downward-facing hole positions the skull atop the spinal column. Other characteristics indicative of bipedalism in Ardipithecus include an increased tarsal region in each foot and a pelvic structure with muscle-to-bone attachment sites comparable to later, bipedal hominins. In addition, the leg bone of Au. anamensis from northern Kenya (4.2–3.9 mya) attests to its bipedalism.
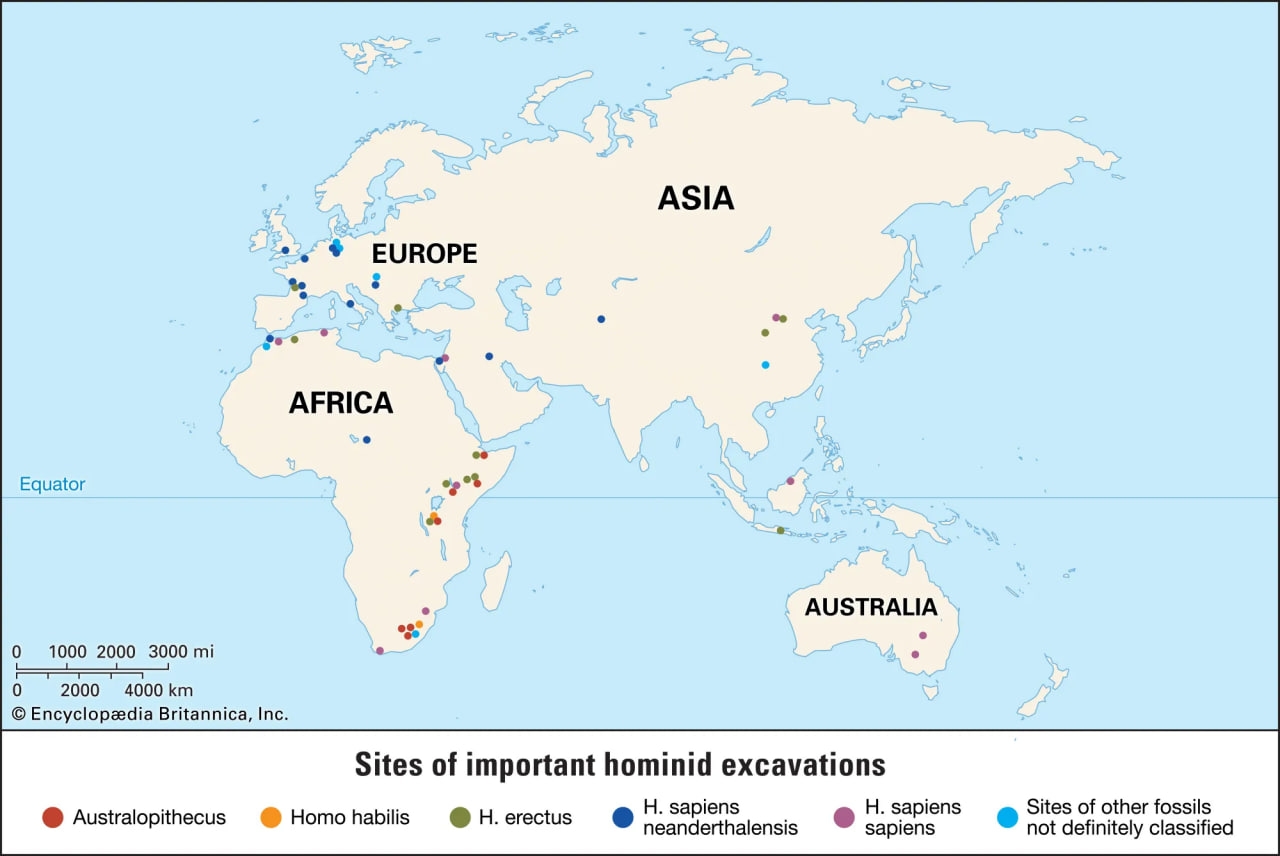
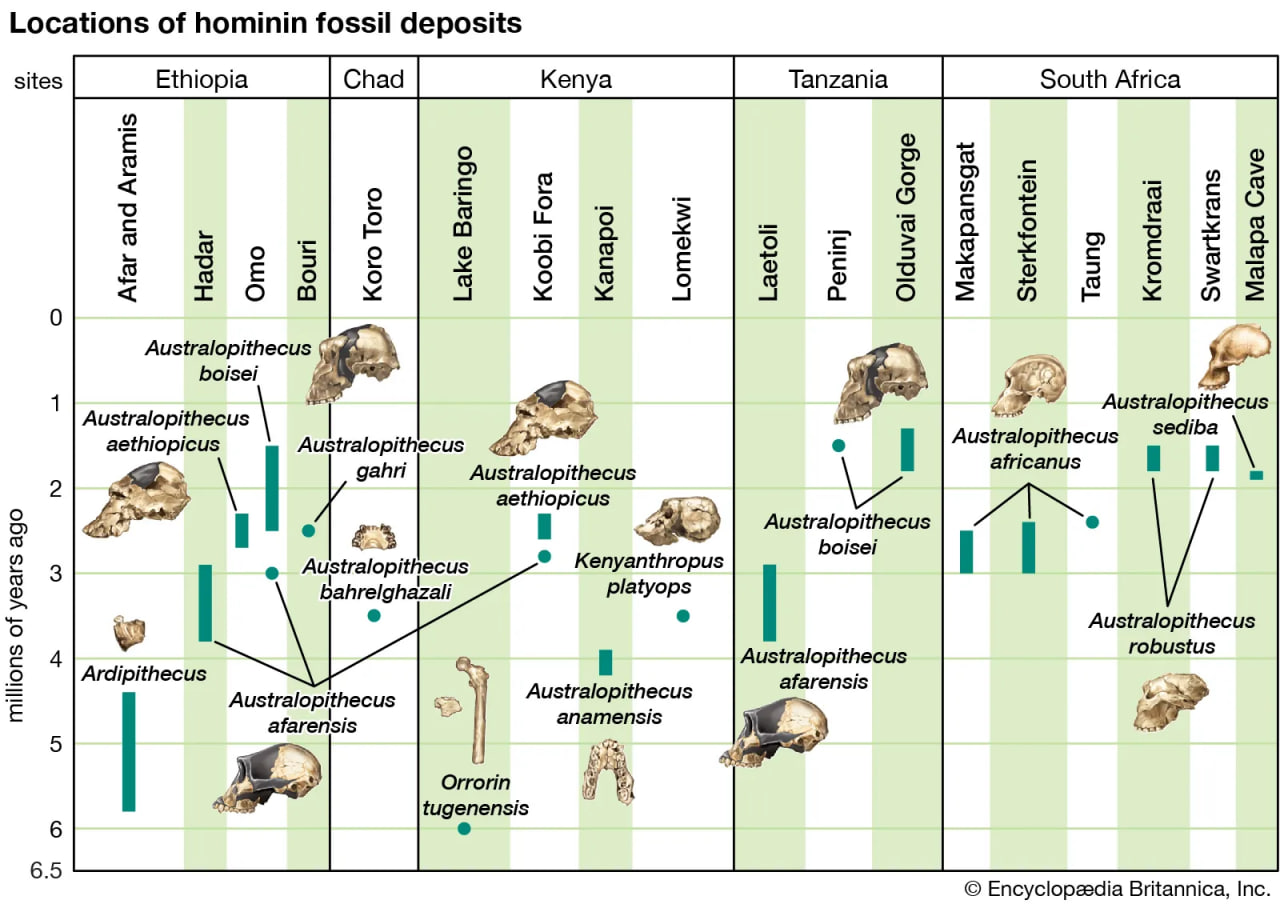
hominid fossil excavation sites
The earliest fossils of human ancestors have been found in Africa.
All hominins living at the time of the Laetoli track makers were probably obligate bipeds when on the ground, but some of them (including some younger species) exhibit features that argue for regular arboreal climbing, probably for food, rest, nightly lodging, and predator avoidance. Hadar, in northern Ethiopia, has yielded a trove of remains of A. afarensis (3.8–2.9 mya). They include many parts of the locomotor skeleton that reveal a bipedal habit: short ilia, a wide and stout sacrum, and femoral angling, among other features. At the same time, the curved fingers and toes, laterally flared ilia, and short femurs with long upper limbs, as well as the configuration of its rib cage, indicate that they could readily climb and maneuver in trees. A. bahrelghazali (3.5–3.0 mya) of central Chad and Kenyanthropus platyops (3.5 mya) from northern Kenya are represented solely by teeth and by skull and jaw fragments from which positional behaviour cannot be inferred.
Parts of the locomotor skeletons of later hominins such as A. africanus (3.3–2.4 mya) and Paranthropus robustus (1.8–1.5 mya) of South Africa do not differ markedly from those of A. afarensis. The locomotor skeleton of eastern African P. boisei (2.2–1.3 mya) is poorly known, but there is no reason to assume that it was different from other Paranthropus species. Bouri, a 2.5-million-year-old site in central Ethiopia, yielded arm and leg bones that are contemporaneous with craniodental remains of A. garhi. The femur is elongated relative to the humerus, as in H. sapiens, but, unlike the human forearm, that of the fossil specimen is relatively long. Thus, by 2.5 mya at least one hominin species had developed the long femurs of striding bipeds, though it retained long forearms like arboreally active Australopithecus and Paranthropus.
H. habilis (2.0–1.5 mya), best known from Olduvai Gorge, Tanzania, exhibits small teeth and a large brain, but it has long upper limbs (especially the forearms), short femurs, curved finger bones, and other chimpanzee-like traits that indicate a mélange of arboreal and terrestrial adaptations. Because of these similarities, some investigators classify H. habilis in genus Australopithecus as Au. habilis.
The pelvis of H. heidelbergensis (600,000–200,000 years ago, or 600–200 kya) and that of Neanderthals (200–30 kya) are distinct from the pelvis of H. sapiens in some features that recall those of Australopithecus. The pelvis is broad, with ilia flaring out to the side. The femoral necks are also relatively long. These features are related to stabilizing the pelvis in stocky bipedal hominins. The pelvises of both H. heidelbergensis and Neanderthals could accommodate a wider birth canal. This feature is important because they may have had notably larger brains (about 1,200 grams [2.65 pounds] and 1,400 grams [3.09 pounds], respectively) than earlier hominins did—a trait that is reflected in the size of the fetal skull.
Regrettably, development of foot structure in early Homo—i.e., between A. afarensis and Neanderthals—is virtually undocumented by skeletal evidence. The oldest footprints indicative of contemporary foot function, however, have been found in Ileret, Kenya. These prints have been dated at 1.51 to 1.53 mya, and their size and depth suggest that they were made by H. ergaster or H. erectus. Therefore, it is safe to assume that by about 1.53 mya the uniquely human locomotor and associated cooling systems were basically established. Subsequent alterations in pelvic shape may be related to the passage of larger-brained babies through the birth canal.
Theories of bipedalism
There are many theories that attempt to explain why humans are bipedal, but none is wholly satisfactory. Increased speed can be ruled out immediately because humans are not very fast runners. Because bipedalism leaves the hands free, some scientists, including Darwin, linked it to tool use, especially tools for defense and hunting—i.e., weapons. This theory is problematic in that the earliest stone artifacts date only to about 3.3 mya, long after hominins had become bipedal, thus requiring an assumption that earlier tools were made of wood or other perishable materials.
Twentieth-century theories proposed a wide array of other factors that might have driven the evolution of hominin bipedalism: carrying objects, wading to forage aquatic foods and to avoid shoreline predators, vigilantly standing in tall grass, presenting phallic or other sexual display, following migrant herds on the savanna, and conserving energy (bipedalism expends less energy than quadrupedism). Furthermore, if the early bipeds were regularly exposed to direct midday tropical sunlight, they would benefit from standing upright in two ways: less body surface would be exposed to damaging solar rays, and they would find relief in the cooler air above the ground.
Some scientists assume that the pre-bipedal primates were terrestrial quadrupeds, perhaps even knuckle-walkers like modern-day chimpanzees, bonobos, and gorillas. Conversely, it is also possible that the first habitual walkers were already well prepared for terrestrial bipedality, having adaptations for running bipedally among branches and boughs, standing upright to forage overhead, and climbing vertical tree trunks and vines. This scenario is suggested by studies of gibbons, which routinely engage in these arboreal activities and virtually never elect to move on the forest floor but, if forced to the ground, run bipedally. Gibbons have relatively long, powerful lower limbs, the same number of lumbar vertebrae that humans have (great apes have fewer), and chests of humanoid configuration. When walking on the ground, gibbons stand up straighter than chimpanzees, which are occasionally bipedal. Moreover, they exert less energy running on the ground than when running bipedally along branches or climbing vertically. Adopting a bipedal stance with full extension of the lower limbs thus would not have been a major challenge, since all apes have this capacity, though there would have been some alteration of the lower limb bones, joints, and ligaments. The foot would probably have gone through the most dramatic change, from a prehensile organ to a heel-supported, propellent one. Increased size and frequent, sustained erect standing on extended lower limbs in order to forage overhanging branches in woodland, thicket, forest edge, and other relatively open habitats would favour the evolution of humanoid hip, knee, and foot structure. While consuming their harvests, bipedal foragers may have squatted often, thereby further selecting for robust heels and for weight distribution between the heel and forefoot and between closely placed feet. Frequent squatting and rising would enhance development of the hamstring, buttock, and anterior thigh muscles (as hip and knee extensors), which are vital for athletic bipedalism. Stretching upward would select for shorter toes and an arched foot. Refinement of the terrestrial bipedal complex probably did not occur until hominins became less dependent upon trees for daytime refuge and other activities and began to forage widely afoot and perhaps to trek seasonally over long distances.
Simply increasing body size would increase locomotor efficiency, because larger animals can more effectively use the elastic energy of tendons and muscles, and they also take fewer strides to cover a given distance than a smaller animal would. Indeed, H. rudolfensis (2.4–1.6 mya), H. ergaster (1.9–1.7 mya), and later species of Homo, including H. sapiens (about 315 kya), are notably taller and heavier than Australopithecus and Paranthropus; however, one species of Homo, H. naledi (the oldest known fossils of which date to 335–200 kya) was comparable in size and weight. There is less size difference between the sexes in Homo species than in many other primates, largely because the females have become larger. Average size in male Australopithecus (41–51 kg [90–112 pounds]) and Paranthropus (40–49 kg [88–108 pounds]) is comparable to that of male chimpanzees (49 kg). The size of females (30–33, 32–34, and 41 kg, respectively) indicates that there was more difference between the sexes (sexual dimorphism) in these hominins than there is in chimpanzees. Sexual dimorphism in H. rudolfensis (60 versus 51 kg [132 versus 112 pounds]) and H. ergaster (66 versus 56 kg [145 versus 123 pounds]) is comparable to that in H. sapiens (58 versus 49 kg [128 versus 108 pounds]).
H. rudolfensis and H. ergaster (1.9–1.5 mya) have long femurs of modern human configuration and internal knee structure like that of H. sapiens; both structures are quite unlike those of chimpanzees and at least some of the smaller tree-climbing primates. This may have been the time also when the distinctive morphology of the human calf muscle (triceps surae) evolved. Unlike those of great apes, it is heavily tendinous, which facilitates its function as an energy-conservant spring during walking and running.
The unique epidermal and respiratory mechanisms of H. sapiens may also have developed in conjunction with regular trekking, sprinting, and endurance running as ancestral Homo secured a foothold in open tropical and subtropical environments. There is a rich concentration of sweat glands in our scalp (apes have few or none in theirs), which helps to cool the head, especially the brain, in high temperatures and during vigorous activity. Postcranially, our abundantly vascular and highly sensitive sparsely haired skin is profusely endowed with sweat glands, whose copious secretions cool an extensive surface by evaporation. The distribution of sweat glands is especially strategic for cooling us while running: there is a greater concentration of sweat glands on the front surfaces of the torso and limbs, against which the air passes as we move forward. Consequently, unlike hairy quadrupeds, we do not have to pause to pant in order to avoid overheating. Furthermore, unlike the chests of quadrupeds, those of humans are freed from the stresses of supporting body weight, necessarily coupled with exhalation in running quadrupeds. We can therefore alter our breathing patterns while moving at various speeds, thereby regulating energy expenditure.
H. ergaster (1.9–1.5 mya), an African species, is the earliest hominin documented with a human thoracic shape. (This species is classified by some paleoanthropologists as an African subgroup of H. erectus.) The thorax of Neanderthals (H. neanderthalensis) is also essentially like that of H. sapiens, but those of other species of Homo are not known.
Hominin habitats

The section Background and beginnings in the Miocene describes certain global climatic changes that reduced forested areas and induced more open terrestrial biomes during the late Miocene Epoch (11.2–5.3 mya). During the succeeding Pliocene Epoch (5.3–2.6 mya) these changes only intensified. In Africa, primates diversified. In Eurasia, contrarily, hominins disappeared by the beginning of the Pliocene. The only descendants of Late Miocene primates in Asia are the extinct Early-Middle Pleistocene Gigantopithecus blacki of southern China and northern Vietnam and the present-day orangutans and gibbons of South and Southeast Asia.
It is reasonable to expect that the increased variety and shifting distribution of African biomes stimulated new hominin lifeways, some of which led to survival and others of which did not. Insofar as habitats have been (or can be) discerned from evidence found with the Pliocene hominin species, hominins inhabited a variety of biomes in eastern, central, and southern Africa. In central Ethiopia, Ar. ramidus is associated with faunal and floral remains indicating a woodland habitat. Later remains, in northern Ethiopia, indicate Au. afarensis inhabited a mosaic of riverine forest, lowland woodland, savanna, and dry bushland. In northern Kenya Au. anamensis lived in dry open woodland or bushland with a gallery forest along a nearby river. In central Chad the northernmost and westernmost species, Au. bahrelghazali, appears to have lived in a mosaic of open and wooded biomes near a river. Mammalian fossils from Lomekwi, northern Kenya, indicate that Kenyanthropus platyops inhabited a relatively well-watered area of forest or closed woodland or the forest edge between them. The habitat of the 3.5-million-year-old Laetoli hominins in northern Tanzania was arguably a mosaic of open grassland and more-closed woodland. The area may have been wetter than it is now. No permanent water source has been identified for the Laetoli area during the Pliocene. Later in the Pliocene, Au. garhi was active on broad, grassy plains bordering a lake in central Ethiopia. Models of the habitat of Au. africanus, based on fauna from the two major South African cave sites—Sterkfontein and Makapansgat—stress closed-canopy wooded conditions: either dry woodland with grasslands nearby or subtropical forest. During the tenures of H. habilis and P. boisei at Olduvai Gorge, northern Tanzania, the climate changed from moist to dry and again to moist before a long dry span that began two million years ago. Specimens of both of these Olduvai hominins are mostly from the shore of an ancient saline, alkaline lake. At Koobi Fora, northern Kenya, specimens of H. habilis have been more commonly found in lake-margin deposits, while those of P. boisei are equally common in river and lake-margin sediments. Fossil pollen indicates that highland forest was nearby and that near the lake there were grassy areas and dense woodland and shrubland
At Konso, southern Ethiopia, P. boisei lived in a grassland habitat. Elsewhere in eastern Africa, P. aethiopicus was associated with closed habitats. The South African cave sites (Swartkrans, Kromdraai, and Drimolen) of P. robustus are associated with open and even arid habitats, but these may not reflect its actual foraging preference.
One of the more profound effects of Pliocene habitat changes was honing the energy-conservant bipedal stride at the time that Homo species deployed out of Africa and into Eurasia. Shortly after Homo evolved in Africa, some species ventured to temperate biomes in Eurasia and then to subtropical and tropical biomes in South and Southeast Asia. Subsequently there was a migration back to Africa, perhaps as early as 1.8–0.9 mya. This hemispheric dispersion of Homo is associated with elaboration of stone tool kits, increased brain size, and reduction in size of the jaws and teeth—all of which are the subject of the next section.
Tools, hands, and heads in the Pliocene and Pleistocene
Refinements in hand structure
power and precision grips
A fully opposable thumb gives the human hand its unique power grip (left) and precision grip (right)
Sharp-edged stones, even small flakes, would be a boon to early hominins who learned how to select and make them for cutting hides, meat, sticks, and other plant material. Stones also would assist in pounding open hard-shelled fruits and nuts, bones for marrow, and skulls for brains. There may have been a span when early hominins used naturally occurring stones and other objects as tools and weapons, much as some wild chimpanzees do today.
Before hominins controlled fire and either built sturdy shelters on the ground or effectively defended caves and rock shelters, they may have constructed platforms in trees for daily activities as well as night lodging. Raw materials, stone hammers, cutting tools, and sticks and stones for defense could be stored in the trees to be used repeatedly. Handheld rocks, clubs, and long stabbing sticks, spears, or other missiles would constitute a formidable defense, especially if employed from the vantage of a tree platform.
Increasing brain size
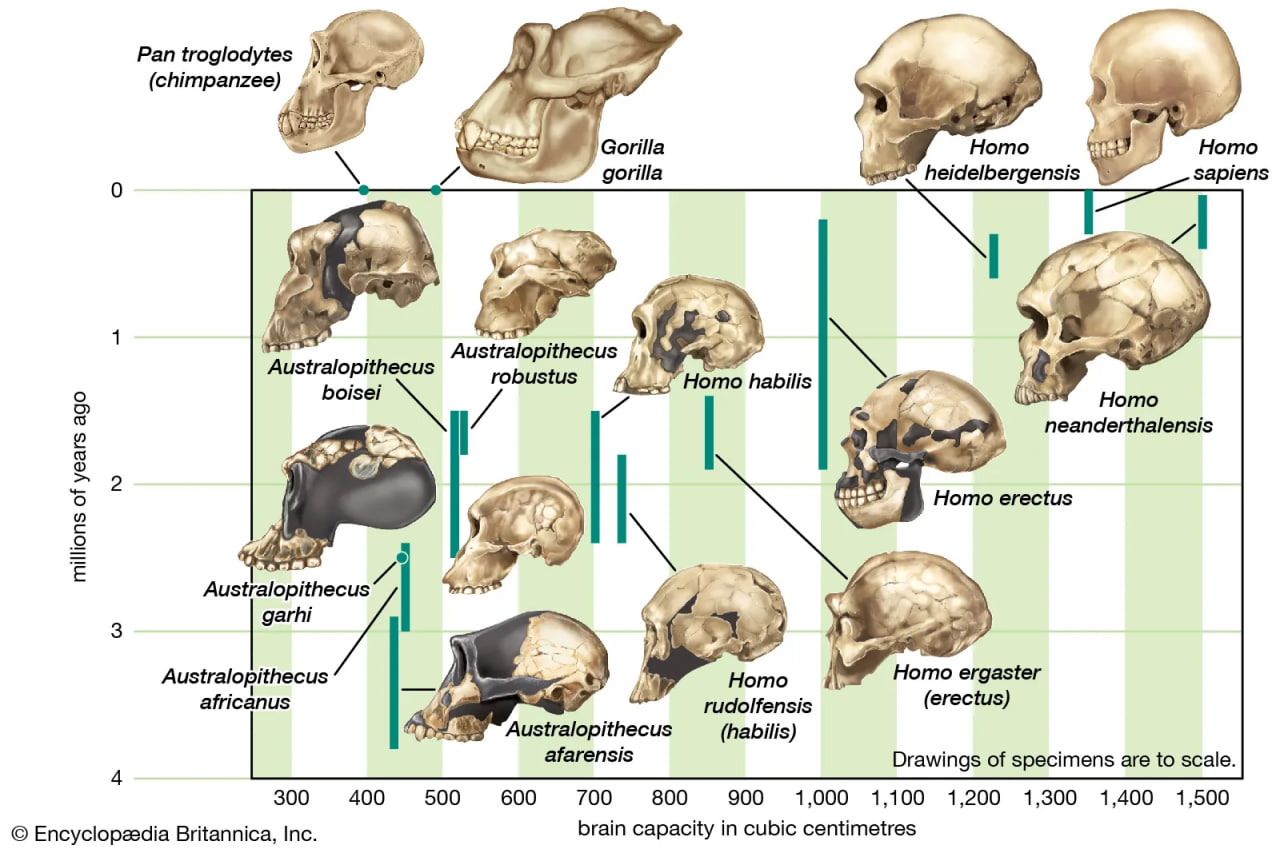
cranial capacity of members of the human lineage
The increase in hominin cranial capacity over time.
Because more complete fossil heads than hands are available, it is easier to model increased brain size in parallel with the rich record of artifacts from the Paleolithic Period (c. 3.3 million to 10,000 years ago), popularly known as the Old Stone Age. The Paleolithic preceded the Middle Stone Age, or Mesolithic Period; this nomenclature sometimes causes confusion, as the Paleolithic itself is divided into Early, Middle, and Late (or Upper) periods. Hominin brain expansion tracks so closely with refinements in tool technology that some scholars ignore other factors that may have contributed to the brain’s increasing size, such as social complexity, foraging strategies, symbolic communication, and capabilities for other culture-mediated behaviours that left no or few archaeological traces.
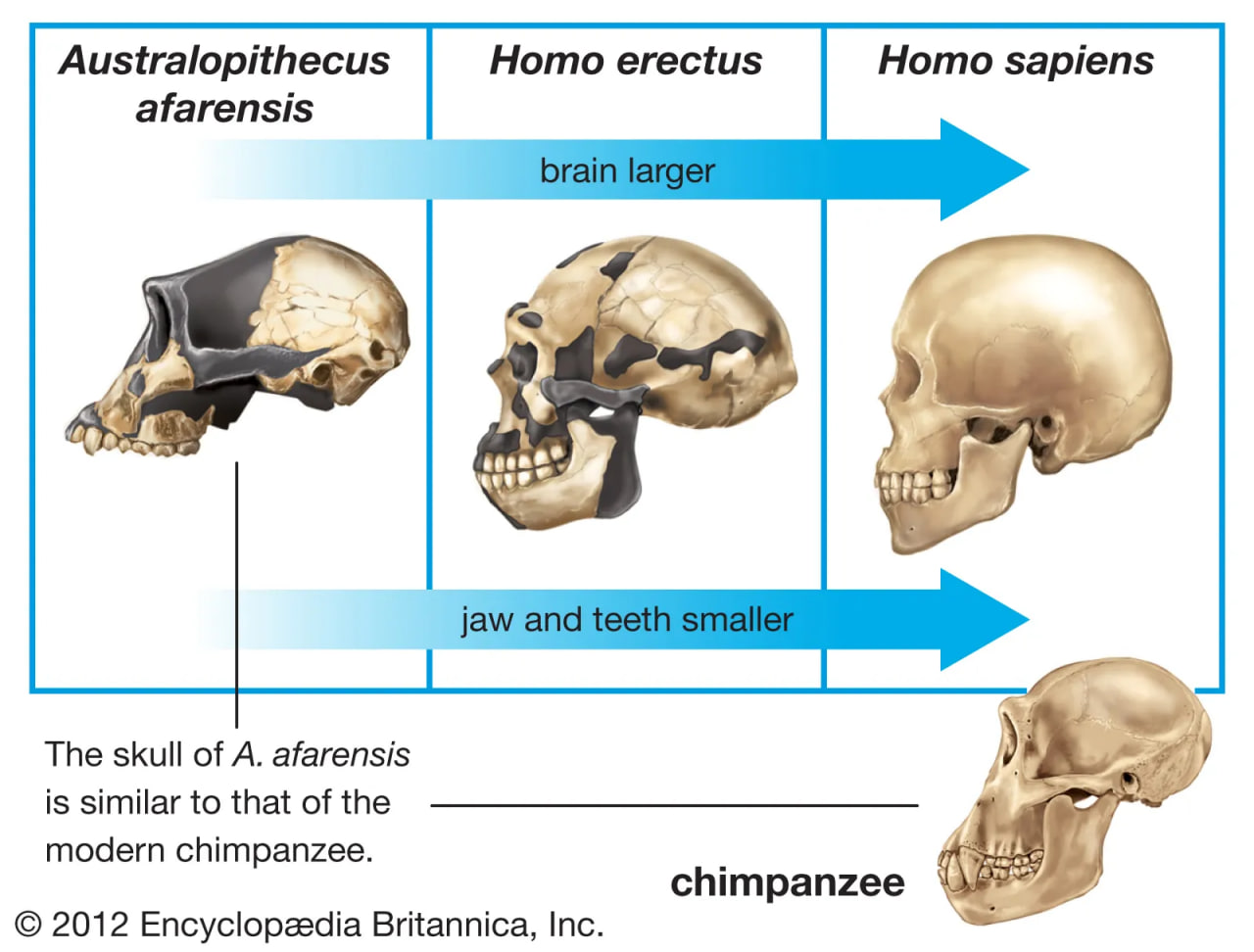
hominin cranial capacity
The evolution of relative cranial capacity and dentition patterns in selected hominins.
Throughout humanevolution, the brain has continued to expand. Estimated average brain masses of A. afarensis (435 grams [0.96 pound]), A. garhi (445 grams [0.98 pound]), A. africanus (450 grams [0.99 pound]), P. boisei (515 grams [1.13 pounds]), and P. robustus (525 grams [1.16 pounds]) are close to those of chimpanzees (395 grams [0.87 pound]) and gorillas (490 grams [1.08 pounds]). Average brain mass of H. sapiens is 1,350 grams (2.97 pounds). The increase appears to have begun with H. habilis (600 grams [1.32 pounds]), which is also notable for having a small body. The trend in brain enlargement continued in Africa with larger-bodied H. rudolfensis (735 grams [1.62 pounds]) and especially H. ergaster (850 grams [1.87 pounds]).
One must be extremely cautious about ascribing greater cognitive capabilities, however. Relative to estimated body mass, H. habilis is actually “brainier” than H. rudolfensis and H. ergaster. A similar interpretive challenge is presented by Neanderthals versus modern humans. Neanderthals had larger brains than earlier Homo species, indeed rivaling those of modern humans. Relative to body mass, however, Neanderthals are less brainy than anatomically modern humans. Relative brain size of Homo did not change from 1.8 to 0.6 mya. After about 600 kya it increased until about 35,000 years ago, when it began to decrease. Worldwide, average body size also decreased in H. sapiens from 35,000 years ago until very recently, when economically advanced peoples began to grow larger while less-privileged peoples did not.
| hominin | number of fossil examples | average capacity of the braincase (cc) |
|---|---|---|
| Australopithecus | 6 | 440 |
| Paranthropus | 4 | 519 |
| Homo habilis | 4 | 640 |
| Javanese Homo erectus (Trinil and Sangiran) | 6 | 930 |
| Chinese Homo erectus (Peking man) | 7 | 1,029 |
| Homo sapiens | 7 | 1,350 |
Overall, there were periods of stagnation and elaboration in stone tool technology during the Paleolithic, but, because of variations over time and between locations as well as the possibility that plant materials were used instead of stone, it is impossible to link brain size with technological complexity and fully human cognitive capabilities. Moreover, in many instances it is impossible to identify assuredly the hominin species that commanded a Paleolithic industry, even when there are associated skeletal remains at the site.
The unreliability of brain size to predict cognitive competence and ability to survive in challenging environments is underscored by the discovery of a distinctive human sample, dubbed H. floresiensis, in a limestone cave on Flores Island, Indonesia, in 2004. The diminutive H. floresiensis had brains comparable in mass to those of chimpanzees and small australopiths, yet they produced a stone tool industry comparable to that of Early Pleistocene hominins and survived among giant rats, dwarf elephants, and Komodo dragons from at least 38 kya to about 18 kya. If they are indeed a distinct species, they constitute yet another archaic human (in addition to H. neanderthalensis, the Denisovans [known from remains from Denisova Cave in Russia], and perhaps H. erectus) that lived contemporaneously with modern humans during the Late Pleistocene.
Refinements in tool design

Replica stone tools of the Acheulean industry, used by Homo erectus and early modern humans, and of the Mousterian industry, used by Neanderthals. (Top, left to right) Mid-Acheulean bifacial hand ax and Acheulean banded-flint hand ax. (Centre) Acheulean hand tool. (Bottom, left to right) Mousterian bifacial hand ax, scraper, and bifacial point
In Africa the Early Paleolithic (3.3–0.2 mya) comprises several industries. The first tools (hammers, anvils, and primitive cutting tools) made way for the earliest human-made chipped flake tools and core choppers (2.5–2.1 mya). Double-faced hand axes, cleavers, and picks (collectively known as bifaces) appeared about 1.5 mya and persisted until about 200 kya. Archaeologists have detected some improvements of technique and product during the half-million-year span of core-flake industries. Although the major biface industry—the Acheulean—has been characterized as basically static, it too shows evidence of refinement over time, finally resulting in elegant, symmetrical hand axes that required notable skill to make.
By 1.8 mya a population of H. erectus lived in Eurasia at what is now Dmanisi, Georgia. The associated choppers, chopping tools, flakes, and scrapers recall the Oldowan core-flake industry of eastern Africa, but there are no bifaces among them. The braincase of the two Dmanisi specimens is smaller than that of African H. ergaster. New geochemical dates for classic hominin localities in Java indicate that H. erectus may have lived in Southeast Asia 1.5 mya, but no industry is certainly identified with them.
El ʿUbeidīya, Israel, provides evidence that people and bifaces had spread out of Africa by 1.4 mya. In Europe, Acheulean tools appear 500 kya and persist until about 250–150 kya; they also occur in South Asia. Sites in China (800 kya), Korea, and Japan contain bifaces, but they differ from Acheulean tools. No such technology has been found in tropical Southeast Asia, where bamboo tools may have sufficed.
In both Africa and Eurasia the Middle Paleolithic was long thought to have lasted from about 200 kya to as recently as 30 kya, depending upon location. While tools from the Early Paleolithic slowly changed across space and time, the Middle Paleolithic was characterized by an explosion of local and regional variations in size and shape and by frequencies of reshaped flakes, blades, scrapers, hand axes, and other tools. Projectile points began to be emphasized in some regions, with bone being used as well as stone; bone arrow points dating to more than 60,000 years ago have been found at Sibudu Cave in South Africa.
Although they vary across time and space, Middle Paleolithic tools as a whole are characterized by carefully prepared cores from which elegant flakes or blades were struck. Notably, tools of this type have been found at the Gademotta site in Ethiopia’s Rift Valley in stratigraphic levels that date to approximately 275 kya. Additional blades dating to roughly 315 kya have been found at Morocco’s Jebel Irhoud site.
Late Paleolithic industries dating to 50–10 kya comprise diverse blade and microblade tools, especially in Europe. Late Paleolithic peoples used a variety of materials for their tools and bodily ornaments, including bone, stone, wood, antler, ivory, and shell. Stone blades were long, thin, and very effective cutting tools. Often, when they became dull, someone retouched them via pressure flaking, which requires fine motor control and coordination. Microblades and other points were probably hafted to produce throwing and stabbing spears. Other composite tools of the period include atlatls, harpoons, fish weirs, and bows and arrows. Late Paleolithic people also developed techniques for grinding and polishing, with which they made beads, pendants, and other artistic objects. They also made needles (perhaps for sewing fitted clothing), fish hooks, and fish gorges.
Reduction in tooth size
The combined effects of improved cutting, pounding, and grinding tools and techniques and the use of fire for cooking surely contributed to a documented reduction in the size of hominin jaws and teeth over the past 2.5 to 5 million years, but it is impossible to relate them precisely. It is not known when hominins gained control over fire or which species may have employed it thereafter for food preparation, warmth, or protection against predators. It is very difficult to discern whether a fire was deliberately produced by hominins or occurred naturally. For example, in a wildfire, burned-out tree stumps might leave circular accumulations of charcoal residue that could be mistaken for hearths, whereas campfires built by mobile hominins would leave no lasting evidence.
Concentrations of charcoal, burned bones, seeds, and artifacts in China and France suggest that H. erectus, H. heidelbergensis, or both used fire as early as 460 kya. Certainly some Middle and Late Paleolithic peoples controlled fire, but hearths are rare until 100 kya. If claims for control of fire in South Africa 1.5 mya are confirmed, P. robustus or H. ergaster would be the first fire keepers.
At first glance early hominin skulls appear to be more like those of apes than humans. Whereas humans have small jaws and a large braincase, great apes have a small braincase and large jaws. In addition, the canine teeth of apes are large and pointed and project beyond the other teeth, whereas those of humans are relatively small and nonprojecting. Indeed, human canines are unique in being incisorlike, and the front lower premolar tooth is bicuspid. In apes and in many monkeys, however, the lower premolar is unicuspid and hones the upper canine tooth to razor sharpness.
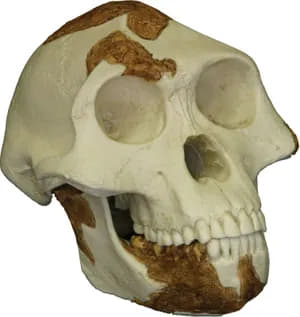
replica skull of Lucy
Australopithecus species also had large rear teeth, but their faces were more protruding because the incisors and canines were not as reduced as those of Paranthropus. Over time the rear teeth progressively increased in size from A. anamensis to A. africanus and H. habilis, with A. afarenis intermediate between A. anamensis and the younger species of Australopithecus. When compared with estimated body size, the pattern of increased tooth size over time is confirmed for Paranthropus.
Tooth wear patterns in A. afarensis indicate that it may have stripped vegetal foods by manually pulling them across the front teeth. The robust-skulled Paranthropus may have eaten tougher foods than did gracile-skulled Australopithecus. Additionally, some paleoanthropologists believe that Paranthropus was vegetarian, while A. africanus had more meat in its diet. Dental morphology and wear patterns indicate that in South Africa P. robustus ate hard foods and that Kenyan P. boisei chewed whole pods and fruits with hard coatings and tough seeds, though they probably did not chew quantities of grass seed, leaves, or bone.
Unlike those of Paranthropus and Australopithecus, the teeth of Homo became smaller over time. H. rudolfensis has large rear teeth, even relative to estimated body size, but H. ergaster approaches the modern human condition. Concomitantly, the face of H. rudolfensis is more like that of Australopithecus than H. ergaster. One expects this trend to be related somehow to changes in diet or techniques of food preparation, but evidence to support this link is not available in the archaeological record.
The emergence of Homo sapiens
The relationships among Australopithecus, K. platyops, Paranthropus, and the direct ancestors of Homo are unknown. Because of its early date and geographic location, A. anamensis may be the common ancestor of A. afarensis, A. garhi, K. platyops, and perhaps the Laetoli Pliocene hominins of eastern Africa, A. bahrelghazali of central Africa, and A. africanus of southern Africa. A. afarensis in turn may be ancestral to P. aethiopicus, which begat P. boisei in eastern Africa and P. robustus in southern Africa.

Ledi-Geraru jawbone
Factors indicating H. rudolfensis as ancestral to later species of Homo are its absolute brain size, large body, and lower limb morphology. These features clearly foreshadow younger species of Homo in Africa and Eurasia. However, a mandible discovered in the Ledi-Geraru area of the Awash River valley in 2013 may point toward a different ancestor—one that clearly belongs to the genus Homo. The mandible provides evidence that dental features associated with later Homo (such as smaller teeth and a much-reduced chin) appeared as early as 2.8 million years ago, well in advance of the advent of H. rudolfensis. While some paleontologists have been quick to associate the specimen with H. habilis, others are considering the possibility that it belongs to a new species of Homo.
replica of KNM-ER 3733, a fossil specimen of Homo erectus

Our ancestry becomes no clearer as the candidates are narrowed to Homo species exclusively. Among paleoanthropologists who accept it as a species distinct from H. erectus, H. ergaster is most often proposed as the ancestor of Homo species of the Pleistocene Epoch. H. heidelbergensis may have arisen from H. ergaster, H. erectus, or H. antecessor, and any or none of them could have been ancestors of H. neanderthalensis and H. sapiens. Neanderthal populations, particularly as represented by specimens from western Europe, probably were not ancestral to modern humans.
H. naledi continues to be the subject of much debate. The oldest fossils of this species are only a few hundred thousand years old; however, several of its morphological traits are very similar to those of Australopithecus, and thus many paleontologists suggest that H. naledi evolved in parallel with H. sapiens.
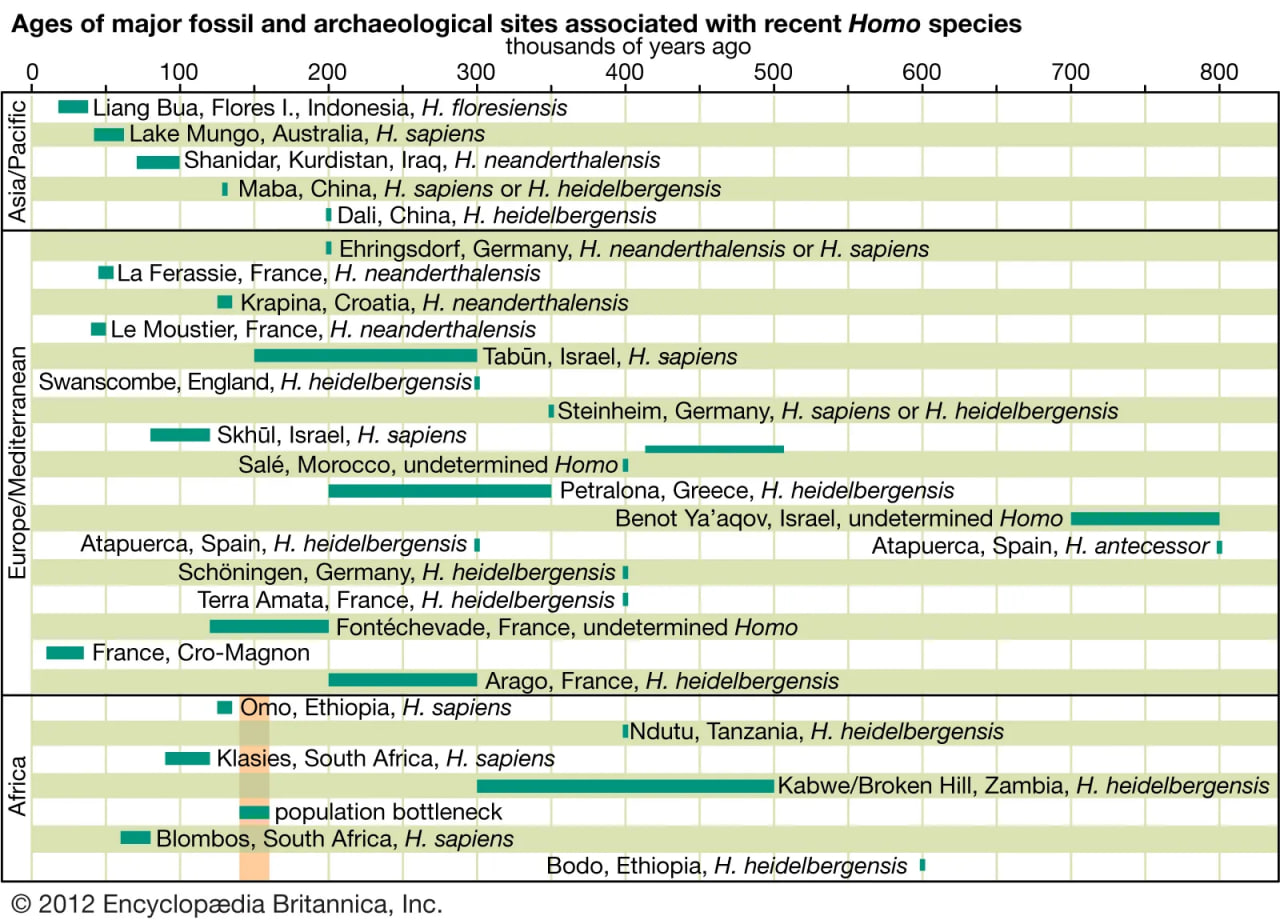
Theorists use fossil remains, genetic traits of modern people around the world, and archaeological and anatomical indicators of cognitive, linguistic, and technological capabilities to support their models of recent human evolution, but no single theory provides definitive resolution of how H. sapiens came to be. The limitations of empirical evidence confound efforts to discern whether distinctive features and lineages developed gradually or over periods of stasis punctuated by rapid change (a theory known as punctuated equilibrium). There are claims for about 20 fossil hominin species over the course of the last six million years, but they are assessed on a case-by-case basis. For example, it appears that Neanderthals (H. neanderthalensis) were a dead end for two ancestral species (H. antecessor and H. heidelbergensis) that changed gradually in Europe from about 700 kya to 30 kya. H. sapiens may have evolved similarly through a series of species represented by African specimens, but other theorists envision a dramatic shift in cognitive capacity and behaviour that qualifies instead as a punctuational change. This change would have occurred in one small African population and would have been followed by a long period of stasis that continues to the present. Such a scenario is not unprecedented, as A. afarensis was a capable biped that appears to have emerged suddenly and persisted for nearly one million years.
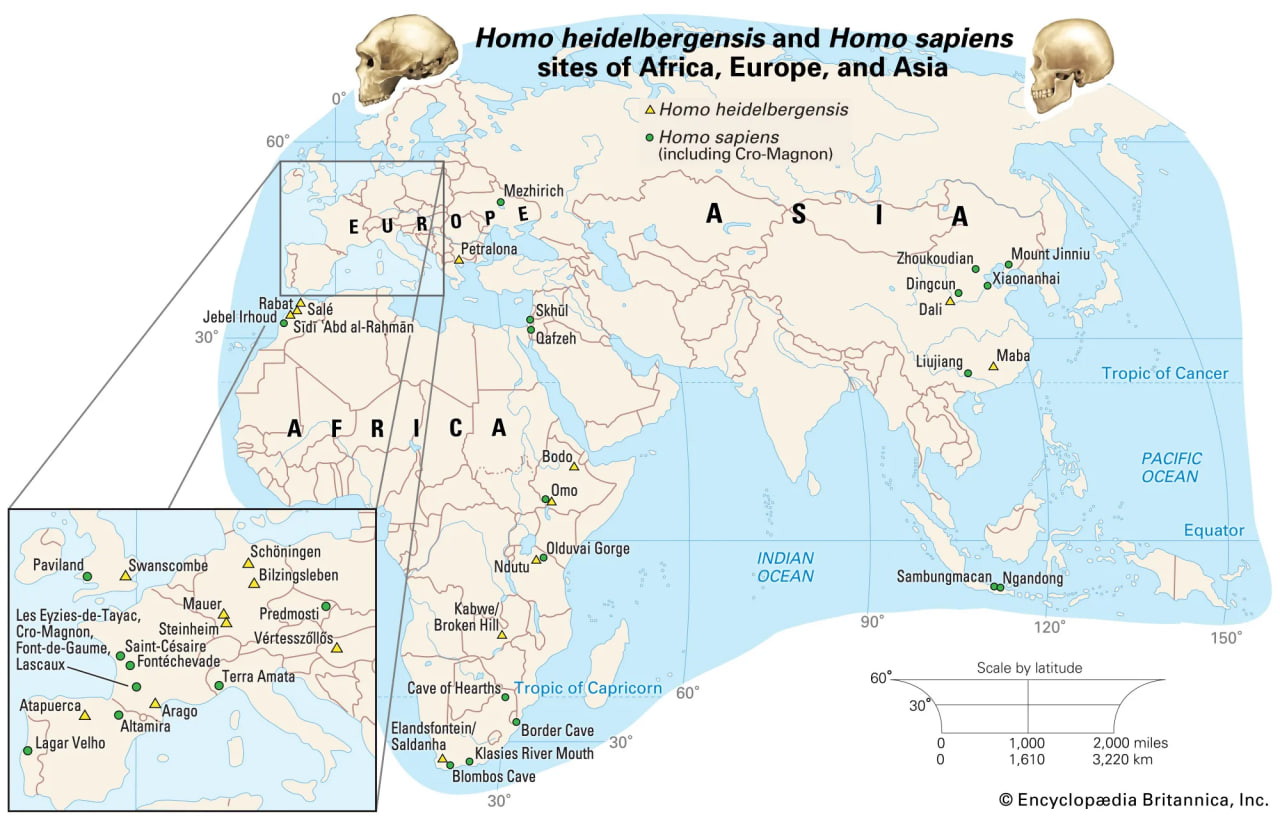
Sites of Homo heidelbergensis and Homo sapiens remains in Africa, Europe, and Asia
There are four basic models that purport to explain the evolution of H. sapiens between about 315 and 30 kya. At one extreme is multiregional evolution, or the regional continuity model. At the other is the African replacement, or “out of Africa,” model. Intermediate are the African hybridization-and-replacement model and the assimilation model. All but the multiregional model maintain that H. sapiens evolved solely in Africa and then deployed to Eurasia and eventually the Americas and Oceania. Both of the replacement models argue that anatomically modern emigrants replaced resident Eurasian and Australasian species of H. sapiens with little or no hybridization. The hybridization-and-replacement model proposes some interbreeding with archaic indigenous populations but with relatively minor effects. Assimilation maintains continuity between archaic and modern humans, most notably in some areas of Eurasia, where gene flow and local selective factors would also produce morphological changes. In this model, unity of the species was maintained by periodic interbreeding across wide areas. Multiregionalists reject the idea that H. sapiens evolved uniquely in Africa. Instead, they advocate that discrete archaic populations of Homo evolved locally in Africa, Asia, and Europe. Throughout their tenures, both the archaic and descendant populations interbred with contemporaries from other areas.
The African replacement model has gained the widest acceptance owing mainly to genetic data (particularly mitochondrial DNA) from existing populations. This model is consistent with the realization that modern humans cannot be classified into subspecies or races, and it recognizes that all populations of present-day humans share the same potential.
uch a tangled line of descent is not surprising given the nomadic lifestyles enabled by bipedalism. There appear to have been successive migrations of hominin species out of Africa, with evolution of new species in Eurasia and occasional migrations back into Africa. For instance, H. ergaster may have been the first hominin to reach Eurasia. Some of its descendants could have moved quickly to East and Southeast Asia, where they begat H. erectus. Others may have evolved into H. heidelbergensis, which populated Europe sparsely and then returned to Africa.
Some paleoanthropologists claim that H. antecessor, found in 800,000-year-old cave deposits at Gran Dolina, Sierra de Atapuerca, Spain, was a direct ancestor of H. neanderthalensis via H. heidelbergensis, which is represented by 300,000-year-old specimens from Sima de los Huesos in the Sierra de Atapuerca. Further, they propose that H. antecessor, from million-year-old deposits in Eritrea, is a direct ancestor of H. sapiens in Africa.
Neanderthals probably evolved in Europe at least partially in response to cold climatic conditions and then migrated to western Asia, where they may have encountered H. sapiens in the Levant. There is no skeletal evidence that they reached the African continent or moved much farther east than Uzbekistan in Central Asia. Features of Neanderthals that argue for adaptation to seasonally frigid biomes include stocky torsos, short limbs (particularly the forearms and legs), and distinctive facial structure. The middle of the face protrudes, the teeth are set forward, the enlarged cheekbones sweep backward, and the nasal passages are voluminous. If Neanderthals wore animal furs and other insulating materials on their heads and bodies while keeping vigorously active in frigid weather, the large nasal chamber would help to cool the blood and prevent overheating the brain, while clothing would reduce the risk of frostbite. The nasal chamber might also conserve moisture during exhalation.
Fossil specimens obtained from the Omo site in Ethiopia (which have been dated to 195 kya) indicate that anatomically modern H. sapiens were present sometime around 200 kya in eastern Africa. The oldest known remains, however, appear at the Jebel Irhoud site in Morocco and date to 315 kya. This evidence suggests that the species might not have emerged in eastern Africa or that it was not confined to the region. Molecular genetic data suggest that early H. sapiens passed through a population bottleneck—that is, a period when they were rare creatures—before rapidly spreading throughout the Old World. H. sapiens migrated to southern China between 120 kya and 80 kya and Europe about 45–43 kya. They replaced indigenous hominin species in Eurasia, and then, as sea levels dropped during glacial periods, adventurous individuals went to sea in watercraft, populating Australia about 65–50 kya and oceanic islands during the most recent 3,000 years. While there is a great deal of evidence pointing to H. sapiens migrating to the Americas by about 14–13.3 kya, the oldest firm evidence places the arrival of H. sapiens in the southwestern United States by 23–21 kya.
Some of the extensive variation in bodily proportions, external features, and blood chemistry of modern peoples may reflect adjustments to biomes over geologically short time spans. However, molecular genetic studies show that genomic differences between even far-flung peoples are minuscule compared with variations within each local population. Accordingly, for modern H. sapiens, race is a mere cultural construct with no biological basis.
Language, culture, and lifeways in the Pleistocene
Speech and symbolic intelligence
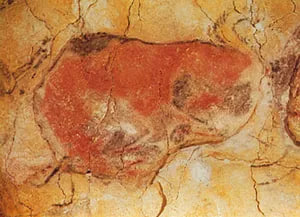
Magdalenian cave painting of a bison
Magdalenian cave painting of a bison, Altamira, Spain.
It is impossible to assess linguistic competency by observing the insides of reassembled fossil craniums that are incomplete, battered, and distorted—and in any case the brains probably did not fit snugly against the walls of the braincase. The apparent cerebral expansion in H. habilis and H. rudolfensis may imply a general increase in cognitive abilities, manipulative skill, or other factors besides speech. Particularly unreliable are claims that the specific internal cranial impressions of a Broca cap is evidence of speech. Prominent Broca caps exist among some chimpanzees, yet no ape has uttered a word, despite laborious attempts to get them to speak.

Cro-Magnon
Conversely, if the theory that different abilities are governed by distinct and separate forms of intelligence (multiple intelligences) is correct, much of tool-using behaviour and artistic ability would have to be based upon neurological structures fundamentally different from those that support verbal ability. Human children begin to use language before they become sophisticated tool users. Similarly, a form of speech might have preceded forms of tool behaviour that are symbolically mediated. Visual arts such as painting and sculpture are expressions of spatial intelligence, which is centred principally in areas of the brain different from those related to speech. Therefore, one cannot expect the problem of language origins or language competence to be clarified by studying Paleolithic symbolism and imagery, despite the awesome array of cave art and polished bone, antler, ivory, stone, and shell artifacts associated with the period. Yet if the stunning proliferation and stylistic variability of tools, bodily ornaments, and artistic works during the Paleolithic do not point unequivocally to the specific use of speech, the presence of these symbolically mediated artifacts—among the earliest of which are shell beads found in Morocco and made about 82,000 years ago—does indicate that early humans were capable of complex conceptual and abstract thought.
Historically, all human groups manifest rich symbolically mediated language, religion, and social, political, and economic systems, even in the absence of elaborate material culture. The demands on the social intelligence of peoples who live in environments with relatively few artifacts are similar to demands placed upon those who depend upon complex technological gadgets and shelters for comfort. Consequently, prehistoric H. sapiens cannot be regarded as cognitively less capable than ourselves, and it is impossible to state which hominin species were “fully human” as symbol users. As a case in point, meticulously documented language studies of captive bonobos and chimpanzees demonstrate that they have the capability to comprehend and use symbols in order to communicate with humans and with one another, but the use of this potential in the wild remains to be demonstrated. Perhaps the human capacity symbolically to represent feelings, situations, objects, and ideas developed before being commandeered by the several intelligences and before it became a boon to vocal communication.
Archaeological evidence indicates that, like at least some of their Pliocene predecessors, the most recent hominins were probably omnivorous, though how much meat was in their diets and whether they obtained it by scavenging, hunting, or both are poorly documented until about 200–100 kya. Stone tools and cut marks on bones at archaeological sites attest to a long history of meat eating in the tribe Hominini, but this practice could have existed long before stone tools were invented. Like chimpanzees, bonobos, baboons, capuchins, and other primates, early Pliocene hominins may have killed and fragmented vertebrate prey with only their hands and jaws instead of tools. The extent to which our ancestors’ hunting, scavenging, or other activities were communal and coordinated via symbolic communication has not been determined.
There is no valid way to estimate group size and composition because there is little evidence of movement patterns, shelters, and graves until the Late Paleolithic. Archaeological traces of human-made shelters occur rarely from 60 kya, then become more common, particularly in regions with notable seasons of inclement weather. The first appearances and development of symbolically based spirituality are also highly elusive because they left no morphological or unarguable archaeological trace until the innovation of writing and ritual paraphernalia; however, there is evidence that Neanderthals used jewelry and other personal ornaments some 44,000 years ago. Although some Neanderthals buried their dead, there is little evidence of mortuary ceremony in their graves. Graves of H. sapiens from 40 kya sometimes contain grave goods.
Learning from the apes
Gorillas, chimpanzees, and bonobos are a rich resource for cultural anthropologists, biologists, and psychologists who speculate on the origins of human society. Gorillas appeal to theorists who stress male dominance and patriarchy. A characteristic gorilla group has one silverback (an older dominant male), one or more subordinate blackback males, adult females outnumbering males, and youngsters of various ages. The silverback is the hub of the cohesive group. Chimpanzee society is also dominated by males, which form a stable core of the group. Chimpanzees and bonobos live in larger groups numbering more than 100 individuals, though they forage, travel, and nest in much smaller bands that vary daily in number and composition. Among chimpanzees there is a top male, followed by several others whose ranks depend upon which other males are present. Bonobos have stronger affiliations between males and females than chimpanzees do, and the organizational hub of bonobo social groups is based on intimate relations among adult females, particularly mothers, which often retain strong bonds with their sons. Adult male bonobos are less strongly bonded with one another than chimpanzee males are. Because bonobos are more pacific and tolerant in social relationships and are highly sexual, they are popular with those who would model our heritage as free of “killer apes.” However, observers of apes, Old World monkeys, and other mammals have documented incidents of aggression as well as concern for others in their subjects. Both tendencies are deeply rooted among the higher primates.
The emergence of the human nuclear family has been a particularly knotty problem for Western evolutionary theorists. Like bonobos and chimpanzees, people probably are fundamentally promiscuous, though such mating behaviour is heavily proscribed by the cultures into which individuals are born and reside. Indeed, theorists who wish to construct models of the emergence of hominin societies on the basis of extant ape societies seldom tackle the overriding fact that humans utilize a wide variety of kinship, social, sexual, and political arrangements, all of which are maintained and expressed symbolically as well as practically. Researchers often fail to search for the cognitive basis of symbolic representation, manipulation, and invention in apes, citing instead forms of behaviour that appear to harbinger specific human conditions. It will take the efforts of several scientific disciplines and sophisticated technology, probably over many years, to discover the underlying nature of our mental faculties, their neurological basis, and their development over time. Apes can play important roles in this enterprise only if they are allowed to survive in their natural habitats and only if they are viewed as being on their own evolutionary paths and not merely as steps toward the human condition.
.


